FAQ's
Need help? Our FAQ section provides quick and easy answers about our products, services, and ordering process.
Still have inquiries?
If you don’t see your question on the list, feel free to reach out to our email address for more information.
Orders are processed and shipped as soon as possible upon receipt of a Purchase Order. Many products are processed or drawn per order only which can affect the number of days. With each Sales Order confirmation, the estimated shipping date is listed
Our team will email the tracking number at the time of label creation. In addition, the tracking number is emailed with each invoice as verification.
You can use the website price and information for your quote if acceptable. Otherwise you can contact us and we will be more than happy to provide you a quote.
A Standing Order is an ongoing order with multiple, defined shipping schedules and quantities. As an alternative, a Blanket Order can be used when schedules and or quantities are unknown.
-Secure pricing over a given period
-Save time by not having all Quotes and Purchase Orders completed one time
-Management of usage demand. No more running out of material
-Invoicing only occurs at the time of each shipment
Contact us and let our customer service team know your needs and that you want to setup a standing order. When placing a standing order the following information will be needed
- Purchase order number or other method of payment
- Shipping and billing addresses
- Items requested
- Quantity of each item requested per shipment
- Schedule of shipment dates
We accept the following forms of payment
- Purchase Order
- All Major Credit Cards
- Wire Transfer
- ACH
You can submit an order to us several different ways. You can fax, email, call or place an order online. Use the information below when wanting to place an order:
Phone: 248-896-0145 or 888-660-6866
Fax: 248-896-0149
Email: sales@innov-research.com
Innovative Research goes to great lengths to ensure that our human sera are the most consistent products commercially available. Our human serum is manufactured using raw material according to time-tested protocols; it is possible to perceive differences in the physical appearance of this product from lot-to-lot. This phenomenon can be largely attributed to variation in diet amongst human beings (particularly with respect to dietary fats). Another source for slight differences comes from non-uniform storage conditions and/or handling variations in the laboratory setting. Due to the special sensitivity of this product (as compared to other sera), it is critical that human serum be cared for as recommended by the manufacturer.
Enzyme-linked immunosorbent assay (ELISA) is a test that uses antibodies and color change to identify a substance.
ELISA is a popular format of a "wet-lab" type analytic biochemistry assay that uses a solid-phase enzyme immunoassay (EIA) to detect the presence of a substance, usually an antigen, in a liquid sample or wet sample.
The ELISA has been used as a diagnostic tool in medicine and plant pathology, as well as a quality-control check in various industries.
Antigens from the sample are attached to a surface. Then, a further specific antibody is applied over the surface so it can bind to the antigen. This antibody is linked to an enzyme, and, in the final step, a substance containing the enzyme's substrate is added. The subsequent reaction produces a detectable signal, most commonly a color change in the substrate.
Performing an ELISA involves at least one antibody with specificity for a particular antigen. The sample with an unknown amount of antigen is immobilized on a solid support (usually a polystyrene microtiter plate) either non-specifically (via adsorption to the surface) or specifically (via capture by another antibody specific to the same antigen, in a "sandwich" ELISA). After the antigen is immobilized, the detection antibody is added, forming a complex with the antigen. The detection antibody can be covalently linked to an enzyme, or can itself be detected by a secondary antibody that is linked to an enzyme through bioconjugation. Between each step, the plate is typically washed with a mild detergent solution to remove any proteins or antibodies that are not specifically bound. After the final wash step, the plate is developed by adding an enzymatic substrate to produce a visible signal, which indicates the quantity of antigen in the sample.
Although it is impossible to claim stability data for every possible application and use for human serum, we conservatively estimate that if this product is stored continuously at -20C or colder; it can be stable for 3 years from the date of manufacture. Storage at any other temperature may affect results.
Bovine Serum Albumin (BSA) is a protein-stabilizing agent and Sodium Azide is a preservative that prevents bacterial growth. The carrier protein and preservative are added to increase functionality and longevity of the antibodies. Antibodies supplied with BSA and Azide can be stored at 2-4°C.
Streptavidin has a high affinity for biotin, with Kd = 1 x 10E-15 moles/L (Green, N.M. [1975] Adv. Protein Chem. 291:85). Streptavidin covalently conjugated to a reporter such as a fluorochrome (R-PE or FITC) or an enzyme (HRP or alkaline phosphatase) has utility in detecting, localizing, and quantitating biotin. Bound streptavidin conjugates can be detected either by fluorescence microscopy or by adding color producing enzyme substrate. This technique is especially useful with biotinylated antibodies. The biotinylation of antibodies is, relatively speaking, an easy procedure and the resulting product will continue to bind to its target antigen with a high degree of specificity and avidity. However, biotin itself cannot be readily detected. Biotin becomes highly apparent once streptavidin conjugated to a reporter is added.
Heating inactivates complement. Active complement can participate in cytolytic events, contract smooth muscle, release histamine from mast cells and platelets, and activate lymphocytic and macrophage cells. Applications where heat inactivated serum is recommended include immunological studies and culturing ES cells, insect cells, and smooth muscle cells.
FBS is not pre-aged. When stored at 2 to 8°C, the possibility exists for various proteins and lipoproteins in serum (e.g., cold agglutinins, fibrinogen, vitronectin, etc.) to aggregate, and form either perceptible material or observed turbidity. This should not affect serum performance. We recommend that you store FBS at -20°C and avoid repeated freeze-thaw cycles.
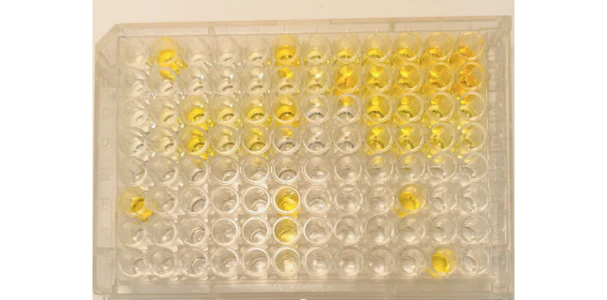
Assay Result: Standard curve in duplicates including zeros (row A and row B), unknowns in duplicates remaining rows
This is an ideal plate/assay turnout
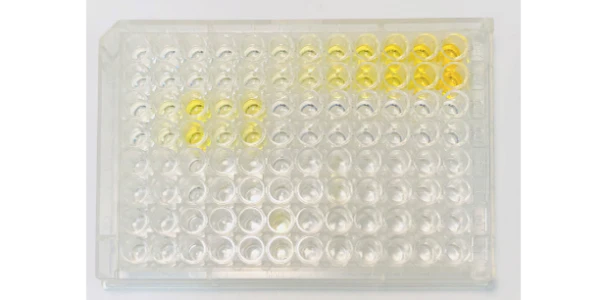
Assay Result: High signal throughout the assay, including the standard curve
Possible Causes:
- One of the most common causes for a plate that shows a high signal in every well is improper washing. To be more specific, it could be due to not using an automated plate washer, not using a manifold dispenser and/or aspirator, not repeating the wash enough, etc. Other causes include not using enough wash buffer or not completely aspirating the wash buffer.
- Another common cause for a high-signal reading is accidentally skipping a step in the kit procedure. Whether it's during washing, reagent preparation, incubation, or anywhere else, it's important that every step is followed exactly as it is written in the kit manual.
- Improper preparation of reagents may also lead to a plate readout with high signal throughout. This may range anywhere from improper dilution concentrations to using the wrong diluent or even adding reagents at an improper time. Again, it's important to follow the assay's instructions exactly as they are written in order to avoid improper readings such as this.
- Finally, a plate with a high signal throughout may have been incubated for too long. It is important that the plate is properly covered and incubated exactly as directed in the instruction manual.
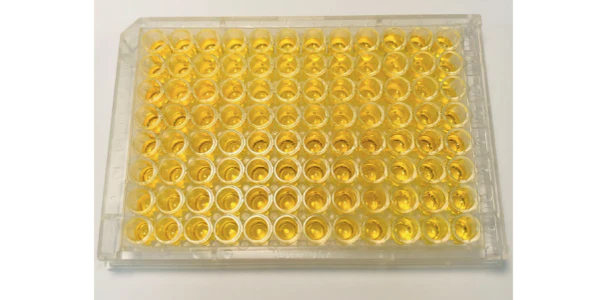
Assay Result: No signal throughout the assay, including the standard curve
Possible Causes:
- One of the most common causes for a plate that shows no signal in every well is improper washing. To be more specific, it could be due to not using an automated plate washer, not using a manifold dispenser and/or aspirator, not repeating the wash enough, etc. Other causes include not using enough wash buffer or not completely aspirating the wash buffer.
- Another cause for a no-signal reading is accidentally skipping a step in the kit procedure. Whether it's during washing, reagent preparation, incubation, or anywhere else, it's important that every step is followed exactly as it is written in the kit manual.
- Improper preparation of reagents may also lead to a plate readout with no signal anywhere. This may range anywhere from improper dilution concentrations to using the wrong diluent or even adding reagents at an improper time. Again, it's important to follow the assay's instructions exactly as they are written in order to avoid improper readings such as this.
- Finally, a plate with no signal throughout may not have been incubated for long enough. It is important that the plate is properly covered and incubated exactly as directed in the instruction manual.

Assay Result: Low precision and/or improper standard curve fit
Possible Causes:
- A common cause for a plate that shows an improper standard curve or low precision is improper washing. To be more specific, it could be due to not using an automated plate washer, not using a manifold dispenser and/or aspirator, not repeating the wash enough, etc. Other causes include not using enough wash buffer or not completely aspirating the wash buffer.
- Improper loading of the wells may also cause low-precision results. This may include, but isn't limited to, splashing, inconsistent volumes in the wells, not changing tips between standard concentrations, and so on.
- If there is insufficient mixing of reagent dilutions you may see low-precision readings or an improper standard curve. This encompasses everything from not reconstituting the standard (primary) completely to not mixing each concentration properly during serial dilutions, and everything in between.
- If there is any sort of dilution error, your assay may return results with an improper standard curve or low-precision results. Common errors include errors when constructing the standard curve, dispensing the wrong volume at a specific step, diluting from a wrong concentration, etc.
Finally, contamination of reagents (wash buffer, diluent) is a potential cause for an error in the standard curve reading or imprecise results. Contamination may occur via improper reagent storage, leaving a reagent bottle open or exposed, leaving the plate uncovered during incubation, and so on.